Chưa được phân loại
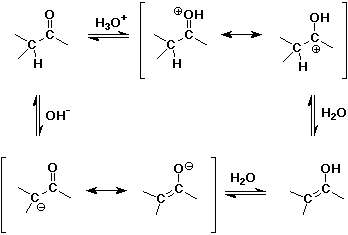
Condensation Reactions
Condensation Reactions
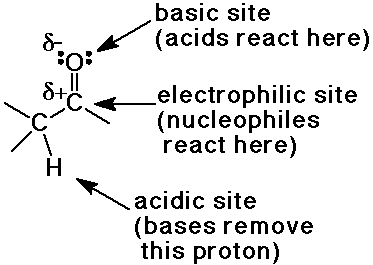
Reactive Sites of the Carbonyl Group
Enols
- tautomers – constitutional isomers that are easily interconverted
enol structure vs. carbonyl (keto) structure differs by location of one H (and double bond)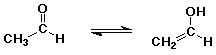
- most carbonyl and carboxyl compounds are in equilibrium with just small amounts of enol
- enol form is favored only in special cases if the C=C double bond or the O-H group is specially stabilized
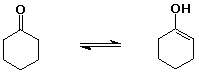
cyclohexanone (1 enol per million ketone molecules)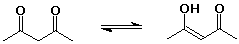
24% 2,4-pentanedione : 76% 4-hydroxy-3-penten-2-one - knowing the right keto-enol forms for the nucleic acid bases allowed Watson and Crick to develop the double-helix concept for DNA
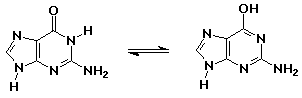
guanine (keto form – as in DNA) // guanine (enol form – aromatic but less stable)
Acid- and Base-Catalyzed Enolization
Alpha-Substitution on Enols
- enols behave like C=C double bonds – react with electrophiles
the net reaction is alpha-substitution
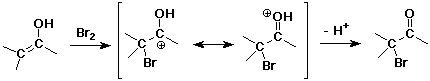
Enolate Anions
- carbonyl compounds have pKa values about 20
- strong bases can deprotonate carbonyl compounds
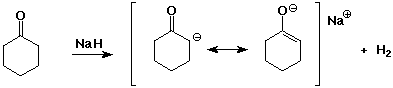
- two carbonyl groups increases the acidity further
pKa of 1,3-diketones is about 9
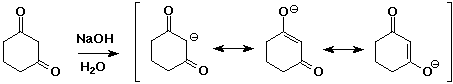
- enolates are good nucleophiles
- enolates are ambident (two-toothed) nucleophiles
usually they react at the C rather than the O anion site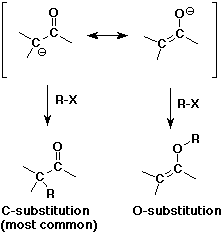
The Aldol Condensation
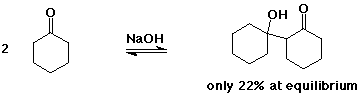
- reaction of an enolate as nucleophile with a carbonyl group,
usually from the same compound
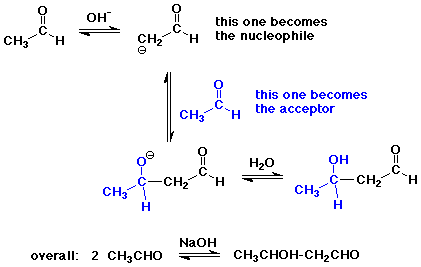
- the reaction is a reversible equilibrium
favored towards product (aldol) only for simple aldehydes
greater substitution favors starting compounds at equilibrium
Dehydration
- the aldol reaction is often combined with dehydration of the initial aldol product, forming a conjugated double bond (an enone)
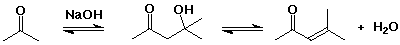
- base-catalyzed elimination via enolate anion (loss of OH-)
- acid-catalyzed elimination via enol (allylic cation intermediate)
Other Aldol Combinations
- crossed aldol reaction – two different carbonyl compounds
works best of one has no alpha-hydrogens, e.g., formaldehyde or benzaldehyde - intramolecular aldol – dicarbonyl compound gives cyclic product
- directed aldol – crossed aldol with one enolate formed in advance
Kinetic vs. Thermodynamic Control
- ketones often can give two different enolate ions – need to control regioselectivity
- more stable enolate (or enol) is the more substituted (thermodynamically favored)
- more rapidly formed enolate is the less substituted (kinetically favored)
- LiN(iPr)2 ( LDA ) irreversibly removes an alpha H from less-substituted side
- to form kinetic enolate, avoid excess ketone (equilibration can occur)
The Claisen Condensation
- condensation with a carboxyl derivative gives substitution instead of addition
- the Claisen condensation gives beta-ketoesters
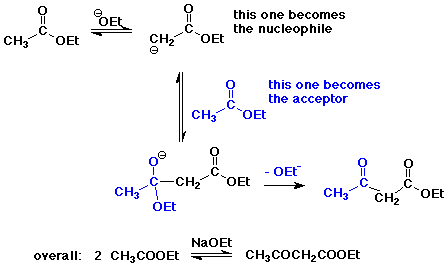
- crossed Claisen reaction – two different esters
works best of one has no alpha-hydrogens,
e.g., formate, carbonate, oxalate, or benzoate ester - Dieckmann condensation – intramolecular Claisen – diester gives cyclic product
Decarboxylation of beta-ketoacids
- Claisen products can be easily hydrolyzed and decarboxylated
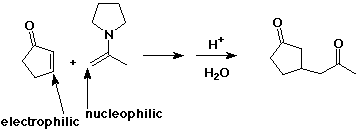
Enamines
- N analog of enols
- formed from 2° amines plus carbonyl compounds
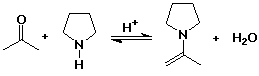
- the N lone pair makes the alpha position electron rich (nucleophilic)
- enamines react with alkyl halides (alkylation) or acyl halides (acylation)
- after hydrolysis, the carbonyl group is reformed
Acetoacetic Ester and Malonic Ester Syntheses
- methods for preparing substituted acetones or acetic acids
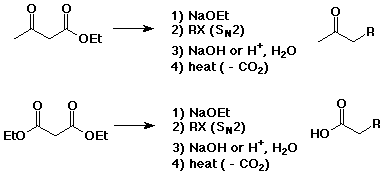
- if steps 1) and 2) are repeated, two substituents can be attached
Michael ( Conjugate or 1,4 ) Addition
- addition of a carbon nucleophile (such as an enolate or enamine)
to the beta-position of a conjugated a,b-unsaturated carbonyl compound,
creating another enolate anion
- Gilman reagents also do conjugate addition
- organolithium and Grignard reagents usually do direct carbonyl addition ( 1,2 addition )
these are rapid, irreversible additions under kinetic control - conjugate addition products (addition to C=C rather than to C=O) are more stable
Other Related Condensation Reactions
- alpha-anions (nucleophiles) can be made next to any good electron-withdrawing group
e.g., nitromethane - these anions can react with carbonyl compounds to give addition
or with carboxyl compounds to give substitution
Biological Aldol and Claisen Reactions
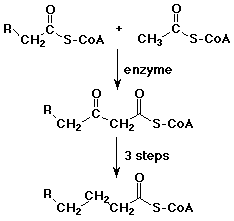
- acetyl Co-A often acts as a biological nucleophile at its alpha-position
- formation of acetoacetyl Co-A is the first step in building up fatty acids
aldolase is an enzyme that adds dihydroxyacetone to glyceraldehyde to form fructose
two 3-carbon sugars join to become a 6-carbon sugar
(Nguồn: http://web.pdx.edu/~wamserc/C335W00/18notes.htm)